
CTC - CT Based Virtual Colonoscopy
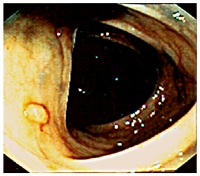
 The colon cancer, which 3 to 5 percent of the population in the developed world will eventually be diagnosed with, is right now the second leading cause of cancer death in the U.S. The cancer grows on the inner surface of the colon and develops from mushroom-like structures, called polyps (see the picture on the left). Most polyps may not become cancerous however those do grow very rapidly, invade and break through the colon wall, or eventually spread to other parts of the body. Size of polyps may vary from 0.3 cm to 3 cm, and with the size increases the risk of becoming cancerous. The only treatment in the pre-cancerous form, or in other words in the polyp stage, is removal of the polyp; early detection and removal of the colonic polyp increases the survival rate.
The colon cancer, which 3 to 5 percent of the population in the developed world will eventually be diagnosed with, is right now the second leading cause of cancer death in the U.S. The cancer grows on the inner surface of the colon and develops from mushroom-like structures, called polyps (see the picture on the left). Most polyps may not become cancerous however those do grow very rapidly, invade and break through the colon wall, or eventually spread to other parts of the body. Size of polyps may vary from 0.3 cm to 3 cm, and with the size increases the risk of becoming cancerous. The only treatment in the pre-cancerous form, or in other words in the polyp stage, is removal of the polyp; early detection and removal of the colonic polyp increases the survival rate. CT colonography (CTC), also known as the virtual colonoscopy, is a minimally invasive technique that uses x-ray CT imaging to examine the interior of the colon, or large intestine. Using the helical CT and computer graphics currently used CT scanners can produce high resolution (<1mm cubic voxel) images in seconds. Conventional examination of these images requires trained radiologists to go over each slice one by one looking for polyps. This is time-consuming and limited by the human factors such as attention span and eye fatigue. Computer aided detection techniques are necessary to increase efficiency and accuracy.
Below Left: Fiberoptic Colonoscopy ; Below Middle: Virtual Colonoscopy; Below Right: Virtual Flythrough (courtesy of Prof. S. Napel, Stanford Univ.)
The VAVlab project "DRESS: Diagnostic Radiology Expert Support Systems (Tubitak Project # 104E035)" has been included in "TUBITAK-ARDEB Success Stories".
Core Collaborators
Computer Aided Polyp Detection using Heat Diffusion Fields (HDFs)
Ender Konukoğlu
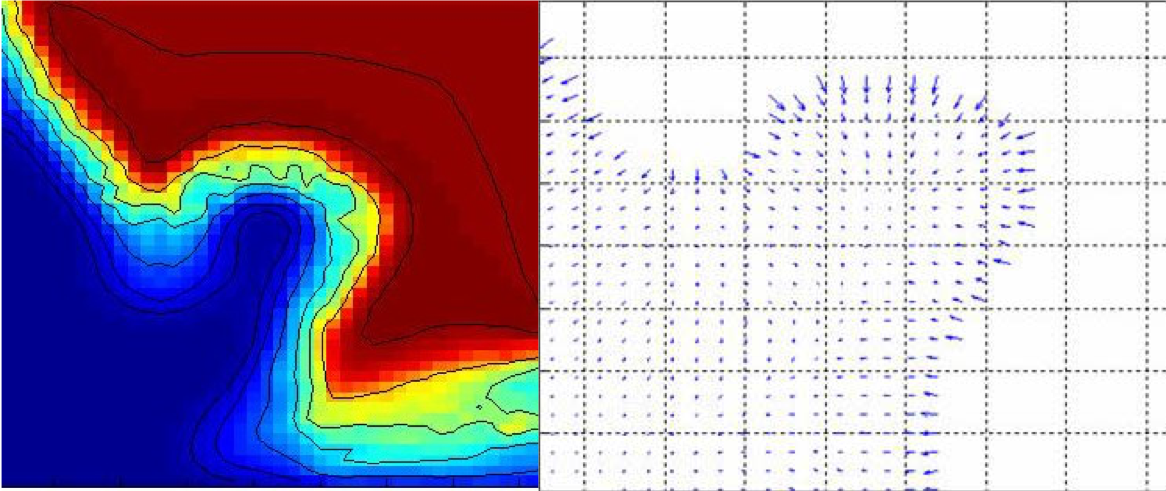
We proposed a nonlinear heat diffusion scheme that would generate sinks with spherically symmetric vector fields (HDFs) near the centers of protruding structures (polyps). The spherical symmetry of the HDFs computed by tracking the iso-temperature surfaces during cooling, increases with the spherical symmetry of the protruding structure. This tendency is exploited in the detection and identification scheme. Roughly, our scheme is based on detecting sinks and classifying them with respect to the spherical symmetry of the HDF at that point. A post-processing is applied to the identified points to increase specificity. This post-processing step makes use of the distribution of air voxels around the detected point (sink). This is achieved via an original spherical sampling approach. This approach is sensitive to the homogeneity of the distribution of air voxels around a given point (sink).
Partially supported by TUBITAK KARIYER-DRESS (104E035) project and by NIH (1R01 CA72023) via Stanford University. Awarded with 3rd place in EUSIPCO 2005 Student Paper Contest. A US Patent has been issued (US7729739 01/06/2010)
Computer Aided Polyp Detection using Edge Displacement Fields (EDFs)
Burak Acar
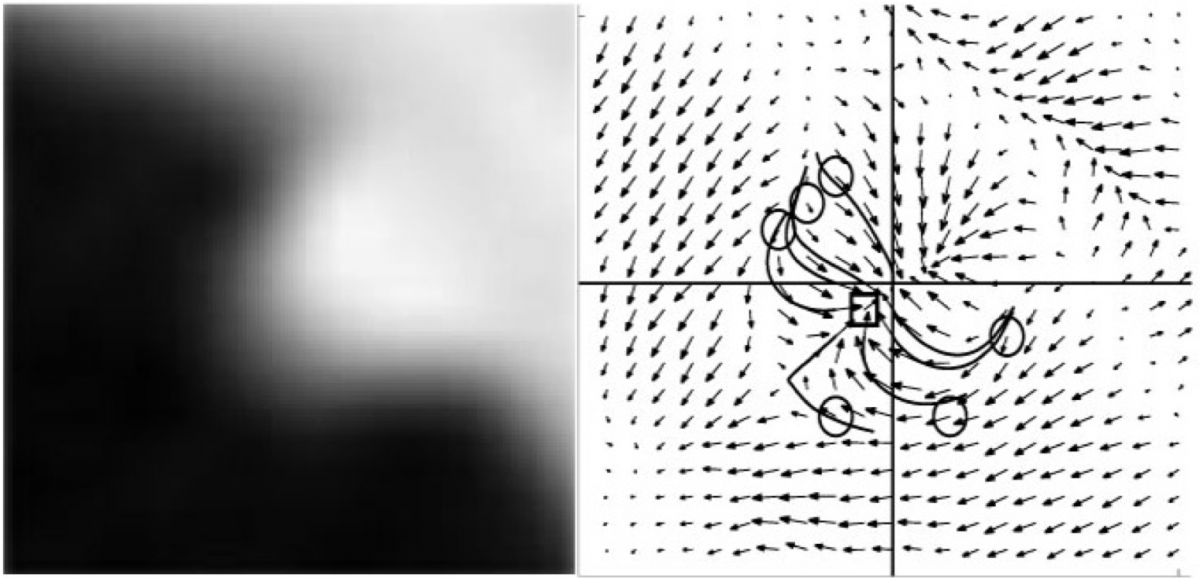
The idea we exploited is to model the way a radiologist reviews 3D CTC images to detect polyps. Radiologists go over 2D images (axial, coronal or sagittal cross-sections of 3D data) one after the other by following the colon lumen visually. As soon as they see a suspicious structure, they scroll back and forth around it. During this process, they unconsciously general a 3D model of the structure there. This is achieved automatically by them as they are trained to do so (to read and interpret radiological images). They are actually making use of the motion of the edges on the 2D image plane as they scroll back and forth. The EDF (Edge Displacement Field) method exploits this point. We developed and evaluated a post-processing algorithm based on this principle. Suspicious structures detected by a pre-processor are extracted. The motion of the edges around these structures as one scrolls back and forth are recorded and represented with a vector field (EDF). The geometry of these vector fields are then used to identify the true polyps, i.e. to decrease the false positive rate of the preprocessor without sacrificing sensitivity.
This research was in part supported by NIH (1R01 CA72023) via Stanford University. A US patent has been issued (US7272251, 18/09/2007)
PELS: Polyp Enhancing Level-Set Methods
Ender Konukoğlu
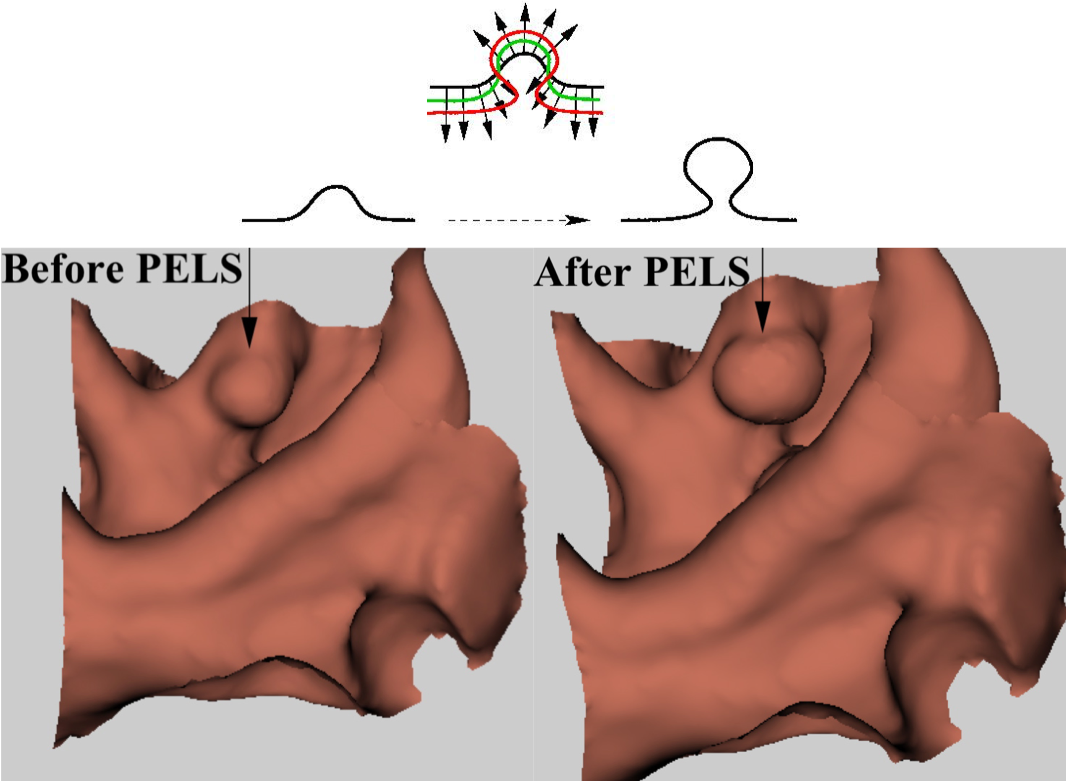
PELS is based on exploiting the idea initially proposed by our group in the HDF method (see above), which was based on enhancing the shapes of polyps via nonlinear heat diffusion fields. The HDF was not designed to change the shapes but rather designed to enhance a certain polyp detector score by making use of the characteristics of polyps shapes. The PELS, on the other hand directly aims at modifying the shape of the colon surface so as to make polyps more distinguishable from other structures, esp. exaggerating the difference between polyps and other structures that mimic polyps. Our approach is based on evolving the colon surface via Level-Set Methods according to some geometrically driven forces to transform true polyps towards more mushroom-like structures. The top figure on the left depicts this evolution. Such an evolution would enhance the performance of almost all polyp detectors as they, in general, rely on the assumption that polyps are nicely protruding hemispherical/spherical structures on the colon wall. The bottom figures show pre and post enhancement colon walls.
Partially supported by TUBITAK KARIYER-DRESS (104E035) project and by NIH (1R01 CA72023) via Stanford University.
Supine-Prone Colon Medial Axis Registration
Burak Acar
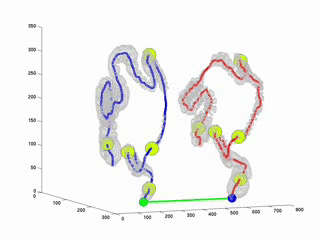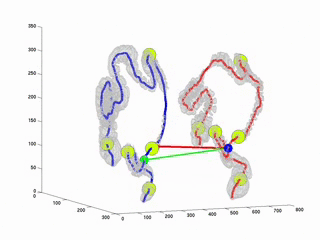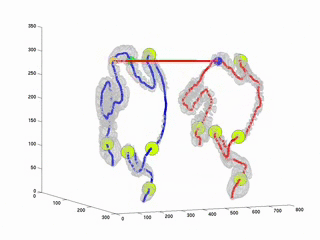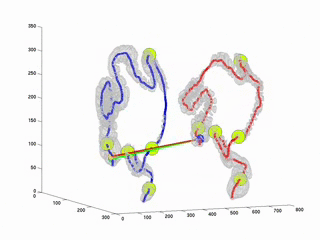
CTC suffers from errors due to imperfect cleansing of the colon. Retained fluid and stool in the colon lumen hides the colon wall, thus degrades polyp detection performance, manual or computer aided. The standard practice against this is to scan the patient twice, in prone and in supine positions and examine the two sets of data in practice. This requires accurate registration of the datasets. Due to the extreme flexibility of the colon wall, this problem has proven to be very difficult. As a first step towards full registration and as a practical solution to the problem, we have considered to register the central axes of the supine and prone colons. Thus, the MDs will be able to accurately locate themselves in parallel in both datasets along the central axes. This will provide a practical and easy way to confirm polyp detections on two datasets. This study is an improvement over our previously proposed algorithm (RSNA2001 & EMBC2001). Here, we used Dynamic Time Warping algorithm where the time is analogous to the path length along the central axis. Experiments on real datasets showed that our algorithm decreases mis-registration (measured along the central axis) from 47 mm to 9mm. Experiments also suggets that the results are independent of initial misregistration.
Partially supported by TUBITAK KARIYER-DRESS (104E035) project, B.A.P. 05M106 and by NIH (1R01 CA72023) via Stanford University. A US patent has been issued (US7224827 29/05/2007).

